I've always been fascinated by this stuff, and was wondering, does it have any use (even for novelties) ?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Oh right lol, I've happened upon a small jar of it. Thought i may be able to put it to some use? 
I remember back in the 80's. When i was on my YTS, the boss had a Daimler Jaguar, and it was used for the light switch on the boot. When it opened, the mercury would short out 2 parts of the switch. 
Steer clear of it . Well clear mate as it is highly toxic and can cause brain damage. Not a lot of people know that fluorescent light tubes have mercury content and have to be disposed of carefully.
Still get them mate mercury tilt switchesI remember back in the 80's. When i was on my YTS, the boss had a Daimler Jaguar, and it was used for the light switch on the boot. When it opened, the mercury would short out 2 parts of the switch.
Cheers, i can do without losing any more brain cells.Steer clear of it . Well clear mate as it is highly toxic and can cause brain damage. Not a lot of people know that fluorescent light tubes have mercury content and have to be disposed of carefully.
I was visualising some sort of perpetual motion gimmick, for getting me off to sleep. (not permanently though) lol
I remember watching a film back in the early 80's called The Exterminator. He was drilling a hole into the end of bullets, and dropping mercury into it, and sealing it up with wax lol. Not really sure what it was supposed to achieve? It looked pretty cool and i was getting excited for the time when he shot someone. But it was disappointing. Didn't seem to have done anything?
Extract from wiki.
Toxicity and safety
See also: Mercury poisoning and Mercury cycle
Mercury and most of its compounds are extremely toxic and must be handled with care; in cases of spills involving mercury (such as from certain thermometers or fluorescent light bulbs), specific cleaning procedures are used to avoid exposure and contain the spill.[93] Protocols call for physically merging smaller droplets on hard surfaces, combining them into a single larger pool for easier removal with an eyedropper, or for gently pushing the spill into a disposable container. Vacuum cleaners and brooms cause greater dispersal of the mercury and should not be used. Afterwards, fine sulfur, zinc, or some other powder that readily forms an amalgam (alloy) with mercury at ordinary temperatures is sprinkled over the area before itself being collected and properly disposed of. Cleaning porous surfaces and clothing is not effective at removing all traces of mercury and it is therefore advised to discard these kinds of items should they be exposed to a mercury spill.
Mercury can be absorbed through the skin and mucous membranes and mercury vapors can be inhaled, so containers of mercury are securely sealed to avoid spills and evaporation. Heating of mercury, or of compounds of mercury that may decompose when heated, should be carried out with adequate ventilation in order to minimize exposure to mercury vapor. The most toxic forms of mercury are its organic compounds, such as dimethylmercury and methylmercury. Mercury can cause both chronic and acute poisoning.
Releases in the environment
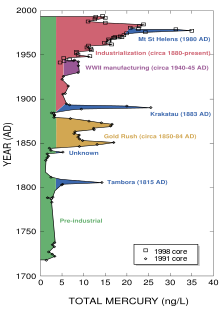
Amount of atmospheric mercury deposited at Wyoming's Upper Fremont Glacier over the last 270 years
Preindustrial deposition rates of mercury from the atmosphere may be about 4 ng /(1 L of ice deposit). Although that can be considered a natural level of exposure, regional or global sources have significant effects. Volcanic eruptions can increase the atmospheric source by 4–6 times.[94]
Natural sources, such as volcanoes, are responsible for approximately half of atmospheric mercury emissions. The human-generated half can be divided into the following estimated percentages:[95][96][97]
Recent atmospheric mercury contamination in outdoor urban air was measured at 0.01–0.02 µg/m3. A 2001 study measured mercury levels in 12 indoor sites chosen to represent a cross-section of building types, locations and ages in the New York area. This study found mercury concentrations significantly elevated over outdoor concentrations, at a range of 0.0065 – 0.523 μg/m3. The average was 0.069 μg/m3.[99]
Mercury also enters into the environment through the improper disposal (e.g., land filling, incineration) of certain products. Products containing mercury include: auto parts, batteries, fluorescent bulbs, medical products, thermometers, and thermostats.[100] Due to health concerns (see below), toxics use reduction efforts are cutting back or eliminating mercury in such products. For example, the amount of mercury sold in thermostats in the United States decreased from 14.5 tons in 2004 to 3.9 tons in 2007.[101]
Most thermometers now use pigmented alcohol instead of mercury, and galinstan alloy thermometers are also an option. Mercury thermometers are still occasionally used in the medical field because they are more accurate than alcohol thermometers, though both are commonly being replaced by electronic thermometers and less commonly by galinstan thermometers. Mercury thermometers are still widely used for certain scientific applications because of their greater accuracy and working range.
Historically, one of the largest releases was from the Colex plant, a lithium-isotope separation plant at Oak Ridge, Tennessee. The plant operated in the 1950s and 1960s. Records are incomplete and unclear, but government commissions have estimated that some two million pounds of mercury are unaccounted for.[102]
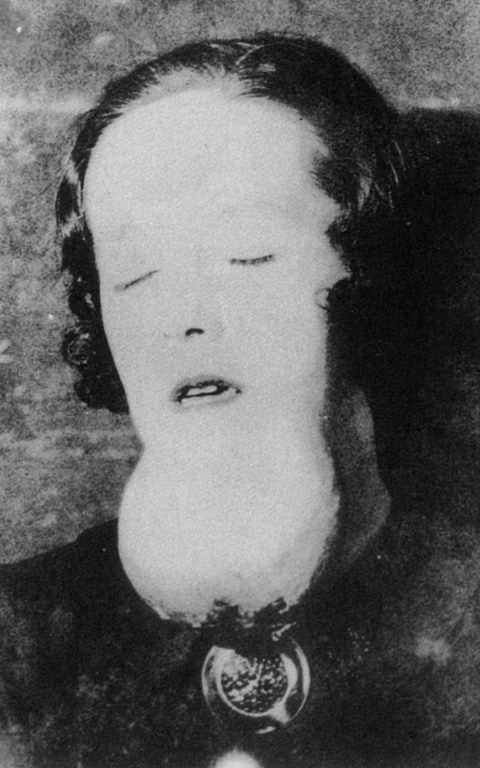
A serious industrial disaster was the dumping of mercury compounds into Minamata Bay, Japan. It is estimated that over 3,000 people suffered various deformities, severe mercury poisoning symptoms or death from what became known as Minamata disease.[103][104]
The tobacco plant readily absorbs and accumulates heavy metals such as mercury from the surrounding soil into its leaves. These are subsequently inhaled during tobacco smoking.[105] While mercury is a constituent of tobacco smoke,[106] studies have largely failed to discover a significant correlation between smoking and Hg uptake by humans compared to sources such as occupational exposure, fish consumption, and amalgam tooth fillings.[107]
Sediment contamination
Sediments within large urban-industrial estuaries act as an important sink for point source and diffuse mercury pollution within catchments.[108] A 2015 study of foreshore sediments from the Thames estuary measured total mercury at 0.01 to 12.07 mg/kg with mean of 2.10 mg/kg and median of 0.85 mg/kg (n=351).[108] The highest mercury concentrations were shown to occur in and around the city of London in association with fine grain muds and high total organic carbon content.[108] The strong affinity of mercury for carbon rich sediments has also been observed in salt marsh sediments of the River Mersey mean of 2 mg/kg up to 5 mg/kg.[109] These concentrations are far higher than those shown in salt marsh river creek sediments of New Jersey and mangroves of Southern China which exhibit low mercury concentrations of about 0.2 mg/kg.[110][111]
Occupational exposure
Due to the health effects of mercury exposure, industrial and commercial uses are regulated in many countries. The World Health Organization, OSHA, and NIOSH all treat mercury as an occupational hazard, and have established specific occupational exposure limits. Environmental releases and disposal of mercury are regulated in the U.S. primarily by the United States Environmental Protection Agency.
Effects and symptoms of mercury poisoning
Main article: Mercury poisoning
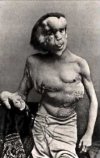
Toxic effects include damage to the brain, kidneys and lungs. Mercury poisoning can result in several diseases, including acrodynia (pink disease), Hunter-Russell syndrome, and Minamata disease.
Symptoms typically include sensory impairment (vision, hearing, speech), disturbed sensation and a lack of coordination. The type and degree of symptoms exhibited depend upon the individual toxin, the dose, and the method and duration of exposure. Case control studies have shown effects such as tremors, impaired cognitive skills, and sleep disturbance in workers with chronic exposure to mercury vapor even at low concentrations in the range 0.7–42 μg/m3.[112][113] A study has shown that acute exposure (4 – 8 hours) to calculated elemental mercury levels of 1.1 to 44 mg/m3 resulted in chest pain, dyspnea, cough, hemoptysis, impairment of pulmonary function, and evidence of interstitial pneumonitis.[114]Acute exposure to mercury vapor has been shown to result in profound central nervous system effects, including psychotic reactions characterized by delirium, hallucinations, and suicidal tendency. Occupational exposure has resulted in broad-ranging functional disturbance, including erethism, irritability, excitability, excessive shyness, and insomnia. With continuing exposure, a fine tremor develops and may escalate to violent muscular spasms. Tremor initially involves the hands and later spreads to the eyelids, lips, and tongue. Long-term, low-level exposure has been associated with more subtle symptoms of erethism, including fatigue, irritability, loss of memory, vivid dreams and depression.[115][116]
Treatment
Research on the treatment of mercury poisoning is limited. Currently available drugs for acute mercurial poisoning include chelators N-acetyl-D, L-penicillamine (NAP), British Anti-Lewisite (BAL), 2,3-dimercapto-1-propanesulfonic acid (DMPS), and dimercaptosuccinic acid (DMSA). In one small study including 11 construction workers exposed to elemental mercury, patients were treated with DMSA and NAP.[117] Chelation therapy with both drugs resulted in the mobilization of a small fraction of the total estimated body mercury. DMSA was able to increase the excretion of mercury to a greater extent than NAP
Toxicity and safety
See also: Mercury poisoning and Mercury cycle
Mercury and most of its compounds are extremely toxic and must be handled with care; in cases of spills involving mercury (such as from certain thermometers or fluorescent light bulbs), specific cleaning procedures are used to avoid exposure and contain the spill.[93] Protocols call for physically merging smaller droplets on hard surfaces, combining them into a single larger pool for easier removal with an eyedropper, or for gently pushing the spill into a disposable container. Vacuum cleaners and brooms cause greater dispersal of the mercury and should not be used. Afterwards, fine sulfur, zinc, or some other powder that readily forms an amalgam (alloy) with mercury at ordinary temperatures is sprinkled over the area before itself being collected and properly disposed of. Cleaning porous surfaces and clothing is not effective at removing all traces of mercury and it is therefore advised to discard these kinds of items should they be exposed to a mercury spill.
Mercury can be absorbed through the skin and mucous membranes and mercury vapors can be inhaled, so containers of mercury are securely sealed to avoid spills and evaporation. Heating of mercury, or of compounds of mercury that may decompose when heated, should be carried out with adequate ventilation in order to minimize exposure to mercury vapor. The most toxic forms of mercury are its organic compounds, such as dimethylmercury and methylmercury. Mercury can cause both chronic and acute poisoning.
Releases in the environment

Amount of atmospheric mercury deposited at Wyoming's Upper Fremont Glacier over the last 270 years
Preindustrial deposition rates of mercury from the atmosphere may be about 4 ng /(1 L of ice deposit). Although that can be considered a natural level of exposure, regional or global sources have significant effects. Volcanic eruptions can increase the atmospheric source by 4–6 times.[94]
Natural sources, such as volcanoes, are responsible for approximately half of atmospheric mercury emissions. The human-generated half can be divided into the following estimated percentages:[95][96][97]
- 65% from stationary combustion, of which coal-fired power plants are the largest aggregate source (40% of U.S. mercury emissions in 1999). This includes power plants fueled with gas where the mercury has not been removed. Emissions from coal combustion are between one and two orders of magnitude higher than emissions from oil combustion, depending on the country.[95]
- 11% from gold production. The three largest point sources for mercury emissions in the U.S. are the three largest gold mines. Hydrogeochemical release of mercury from gold-mine tailings has been accounted as a significant source of atmospheric mercury in eastern Canada.[98]
- 6.8% from non-ferrous metal production, typically smelters.
- 6.4% from cement production.
- 3.0% from waste disposal, including municipal and hazardous waste, crematoria, and sewage sludge incineration.
- 3.0% from caustic soda production.
- 1.4% from pig iron and steel production.
- 1.1% from mercury production, mainly for batteries.
- 2.0% from other sources.
Recent atmospheric mercury contamination in outdoor urban air was measured at 0.01–0.02 µg/m3. A 2001 study measured mercury levels in 12 indoor sites chosen to represent a cross-section of building types, locations and ages in the New York area. This study found mercury concentrations significantly elevated over outdoor concentrations, at a range of 0.0065 – 0.523 μg/m3. The average was 0.069 μg/m3.[99]
Mercury also enters into the environment through the improper disposal (e.g., land filling, incineration) of certain products. Products containing mercury include: auto parts, batteries, fluorescent bulbs, medical products, thermometers, and thermostats.[100] Due to health concerns (see below), toxics use reduction efforts are cutting back or eliminating mercury in such products. For example, the amount of mercury sold in thermostats in the United States decreased from 14.5 tons in 2004 to 3.9 tons in 2007.[101]
Most thermometers now use pigmented alcohol instead of mercury, and galinstan alloy thermometers are also an option. Mercury thermometers are still occasionally used in the medical field because they are more accurate than alcohol thermometers, though both are commonly being replaced by electronic thermometers and less commonly by galinstan thermometers. Mercury thermometers are still widely used for certain scientific applications because of their greater accuracy and working range.
Historically, one of the largest releases was from the Colex plant, a lithium-isotope separation plant at Oak Ridge, Tennessee. The plant operated in the 1950s and 1960s. Records are incomplete and unclear, but government commissions have estimated that some two million pounds of mercury are unaccounted for.[102]
A serious industrial disaster was the dumping of mercury compounds into Minamata Bay, Japan. It is estimated that over 3,000 people suffered various deformities, severe mercury poisoning symptoms or death from what became known as Minamata disease.[103][104]
The tobacco plant readily absorbs and accumulates heavy metals such as mercury from the surrounding soil into its leaves. These are subsequently inhaled during tobacco smoking.[105] While mercury is a constituent of tobacco smoke,[106] studies have largely failed to discover a significant correlation between smoking and Hg uptake by humans compared to sources such as occupational exposure, fish consumption, and amalgam tooth fillings.[107]
Sediment contamination
Sediments within large urban-industrial estuaries act as an important sink for point source and diffuse mercury pollution within catchments.[108] A 2015 study of foreshore sediments from the Thames estuary measured total mercury at 0.01 to 12.07 mg/kg with mean of 2.10 mg/kg and median of 0.85 mg/kg (n=351).[108] The highest mercury concentrations were shown to occur in and around the city of London in association with fine grain muds and high total organic carbon content.[108] The strong affinity of mercury for carbon rich sediments has also been observed in salt marsh sediments of the River Mersey mean of 2 mg/kg up to 5 mg/kg.[109] These concentrations are far higher than those shown in salt marsh river creek sediments of New Jersey and mangroves of Southern China which exhibit low mercury concentrations of about 0.2 mg/kg.[110][111]
Occupational exposure
Due to the health effects of mercury exposure, industrial and commercial uses are regulated in many countries. The World Health Organization, OSHA, and NIOSH all treat mercury as an occupational hazard, and have established specific occupational exposure limits. Environmental releases and disposal of mercury are regulated in the U.S. primarily by the United States Environmental Protection Agency.
Effects and symptoms of mercury poisoning
Main article: Mercury poisoning
Toxic effects include damage to the brain, kidneys and lungs. Mercury poisoning can result in several diseases, including acrodynia (pink disease), Hunter-Russell syndrome, and Minamata disease.
Symptoms typically include sensory impairment (vision, hearing, speech), disturbed sensation and a lack of coordination. The type and degree of symptoms exhibited depend upon the individual toxin, the dose, and the method and duration of exposure. Case control studies have shown effects such as tremors, impaired cognitive skills, and sleep disturbance in workers with chronic exposure to mercury vapor even at low concentrations in the range 0.7–42 μg/m3.[112][113] A study has shown that acute exposure (4 – 8 hours) to calculated elemental mercury levels of 1.1 to 44 mg/m3 resulted in chest pain, dyspnea, cough, hemoptysis, impairment of pulmonary function, and evidence of interstitial pneumonitis.[114]Acute exposure to mercury vapor has been shown to result in profound central nervous system effects, including psychotic reactions characterized by delirium, hallucinations, and suicidal tendency. Occupational exposure has resulted in broad-ranging functional disturbance, including erethism, irritability, excitability, excessive shyness, and insomnia. With continuing exposure, a fine tremor develops and may escalate to violent muscular spasms. Tremor initially involves the hands and later spreads to the eyelids, lips, and tongue. Long-term, low-level exposure has been associated with more subtle symptoms of erethism, including fatigue, irritability, loss of memory, vivid dreams and depression.[115][116]
Treatment
Research on the treatment of mercury poisoning is limited. Currently available drugs for acute mercurial poisoning include chelators N-acetyl-D, L-penicillamine (NAP), British Anti-Lewisite (BAL), 2,3-dimercapto-1-propanesulfonic acid (DMPS), and dimercaptosuccinic acid (DMSA). In one small study including 11 construction workers exposed to elemental mercury, patients were treated with DMSA and NAP.[117] Chelation therapy with both drugs resulted in the mobilization of a small fraction of the total estimated body mercury. DMSA was able to increase the excretion of mercury to a greater extent than NAP
I remember being a kid and my grandad having a bottle from John Browns shipyard. He used to let me play with it but not actually touch it with my hands. I was fascinated by the stuff.
I was a bit surprised to find it vapourises at room temperature :S.
I'd heard this years ago:
Mad as a hatter - Wikipedia
I'd heard this years ago:
Mad as a hatter - Wikipedia
I used to pour a bit from hand to hand LOL.
A friend had a mercury arc rectifier as an ornament and he used to work with them years ago. Said they gave off a "ghostly" light when they were working.
Mercury-arc valve - Wikipedia
A friend had a mercury arc rectifier as an ornament and he used to work with them years ago. Said they gave off a "ghostly" light when they were working.
Mercury-arc valve - Wikipedia
I bought a jam jar of the stuff as a kid from a junk shop, only paid a couple of quid for it. I knew it as dangerous stuff (and quite heavy IIRC) so I never even opened the jar. But I figured it must have been worth a lot more than I paid for it and intended selling it on. Unfortunately it 'disappeared' from my room, I reckon my Mum confiscated it. I wish I'd know about the arc-valve.
Similar threads
- Replies
- 14
- Views
- 1K
- Replies
- 1
- Views
- 318